Laboratory of Coleen M. Atkins, Ph.D.
Therapies to Reduce Inflammation after Traumatic Brain Injury
Circuitry Remodeling in the Chronic Injured Brain
Interaction of Stress and Concussion

THERAPIES TO REDUCE INFLAMMATION AFTER TRAUMATIC BRAIN INJURY
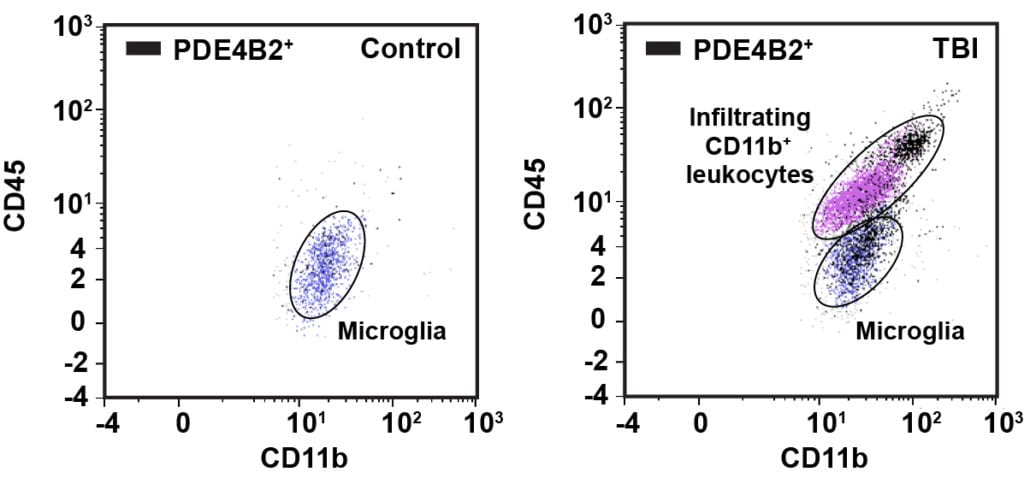
Every year an estimated 1.7 million people suffer a traumatic brain injury (TBI). The initial brain trauma triggers a complex inflammatory response; this acute inflammatory response increases neuronal death and worsens outcome after TBI. The inflammatory response is regulated by a diverse array of molecules; one of these molecules is the highly ubiquitous second messenger, cAMP. Inhibition of a cAMP-hydrolyzing enzyme, phosphodiesterase 4 (PDE4), has been used as an anti-inflammatory strategy in models of systemic inflammation and CNS injury. We have found that the PDE4B isoform, PDE4B2, is elevated acutely after TBI; additionally, we found that PDE4B2 is upregulated in microglia and infiltrating leukocytes at 24 hours after TBI. Currently, we are exploring the anti-inflammatory potential of cAMP elevation after TBI using selective inhibitors of PDE4.
CIRCUITRY REMODELING IN THE CHRONIC INJURED BRAIN
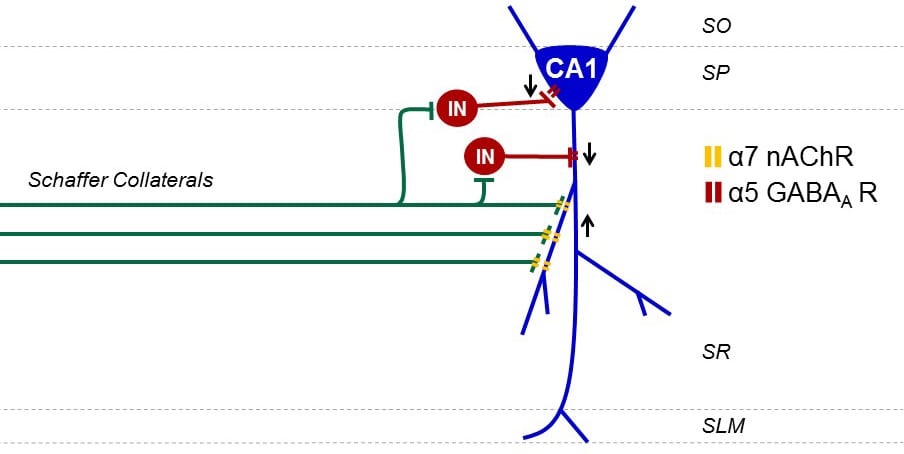
Profound neuronal circuit changes occur within the hippocampus that chronically hinder recovery of cognitive functioning after traumatic brain injury (TBI). Principal changes during the chronic recovery phase of brain injury include a depression in basal synaptic transmission and impaired expression of long-term potentiation (LTP) in area CA1 of the hippocampus. Multiple studies report that normal hippocampal electroencephalograph oscillatory patterns in the 6-10 Hz range are reduced after TBI. These theta rhythms are responsible for arousal, sensorimotor processing and promote LTP expression in the hippocampus to encode spatial memory. The hippocampal theta rhythm arises from cholinergic projections from the medial septum via the fimbria-fornix. Atrophy in the fimbria-fornix is routinely observed in TBI survivors and suggests that the cholinergic tone required for establishing theta rhythm is deficient. These changes are reminiscent of Alzheimer’s disease and age-related mild cognitive impairment where targeting the cholinergic system restores hippocampal theta rhythm and promotes learning and memory in the dysfunctional brain. We are currently testing strategies to restore theta rhythm after TBI and rescue chronic learning and memory deficits. By targeting the cholinergic system, we can improve synaptic transmission, rescue long-term potentiation and chronic learning and memory deficits after TBI.
INTERACTION OF STRESS AND CONCUSSION
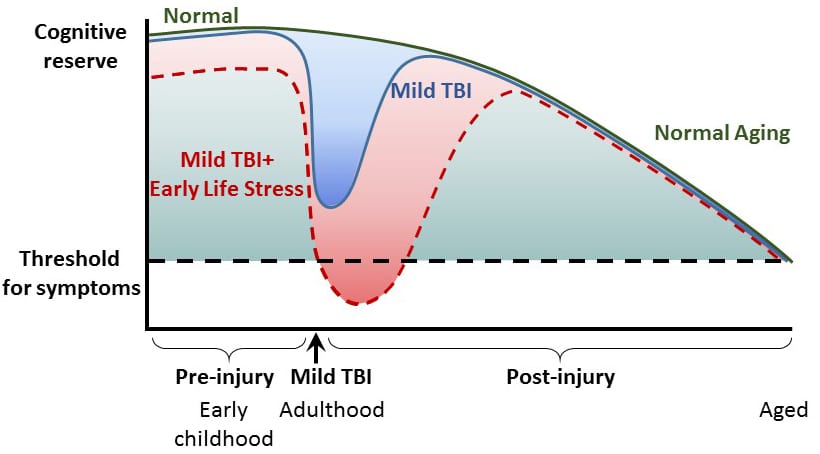
Mild traumatic brain injury (mTBI) and concussion are major clinical problems in the United States. Although the majority of people with mTBI recover within a few weeks, a subset of people have persistent symptoms lasting for months. However, the factors that contribute to developing persistent symptoms after mTBI are unknown. One potential factor recently identified in a study of mTBI patients is pre-exposure to stressful life experiences. Chronic early life stress is highly prevalent in the United States, and a major cause of early life stress in childhood is neglect. Maltreated children are at risk for developing long-term emotional, cognitive and medical problems that emerge during middle age. Using brief daily maternal separation in rat pups to model childhood neglect, we have found that the combination of early life stress with mTBI in adulthood increased pro-inflammatory cytokine expression, worsened cortical and hippocampal atrophy and impaired hippocampal-dependent learning. We are currently identifying the molecular mechanisms that underlie the increase in inflammation and pathology to develop therapeutic interventions to reduce the exacerbation of memory deficits after early life stress and mTBI.
The Atkins laboratory has an exciting opportunity for a Postdoctoral Associate or Associate Scientist position. This position is for an electrophysiologist interested in studying the circuitry changes resulting from traumatic brain injury. The focus of the Atkins’s laboratory is to develop cognitive therapeutics for chronic traumatic brain injury. The candidate will study this problem at the electrophysiology level, using hippocampal slice recordings. If interested, please send your cv to catkins@miami.edu.
